Kyowa Kirin to Start Construction of a New Biopharmaceutical API Manufacturing Building at its Takasaki Plant
May 17, 2022
Tokyo, Japan, May 17, 2022 --Kyowa Kirin Co., Ltd. (President & CEO: Masashi Miyamoto, “Kyowa Kirin”, TSE: 4151) has decided to build a new active pharmaceutical ingredient (API) manufacturing building (HB7 Building) at its Takasaki Plant (Takasaki City in Gunma Prefecture, Plant Manager: Ryuji Nomura) that is responsible for manufacturing biopharmaceuticals.
The HB7 Building will manufacture APIs for biopharmaceuticals utilizing Kyowa Kirin’s unique antibody technology and protein engineering, and it will be equipped with both a GMP*1 compliant manufacturing facility and pilot facility for investigational APIs in early phase development. Because both facilities are planned to have the same single-use manufacturing equipment, it will be possible to use the same equipment configuration for every step from the initial process development for biopharmaceutical API manufacturing to the manufacturing of the investigational API. This will enable even quicker supply of investigational APIs, and it is ultimately expected to facilitate a rapid start of early phase clinical trials. Also, through the construction of the HB7 Building, Kyowa Kirin will possess its own manufacturing facility for early phase development, enabling more flexible high-mix, small-lot manufacturing of products in early phase development. Moreover, it is planned to use the pilot facility to validate continuous production system*2, which is new technology for biopharmaceutical APIs, as well as utilize the facility for technological innovation directed at the stable supply of biopharmaceuticals in the future.
In addition, it is planned to equip a new facility in the new building that enables hands-on training with same equipment used in GMP manufacturing. By providing training based on individual skill levels to the plant staff and operators using actual manufacturing equipment, Kyowa Kirin aims to continuously foster excellent personnel. Also, through the acceleration of digital transformation, further automation of the manufacturing process will be promoted, and data integrity will be enhanced. At the same time, the building will be a next generation manufacturing facility with low environmental impact, including the installation of solar panels and productive use of waste heat.
The investment in the HB7 Building is planned to above ¥10 billion. Construction is planned to be completed in April 2024, and the facilities will be successively brought online from June 2024 as preparations are complete. Manufacturing of investigational APIs is planned to start from 2025 onwards.
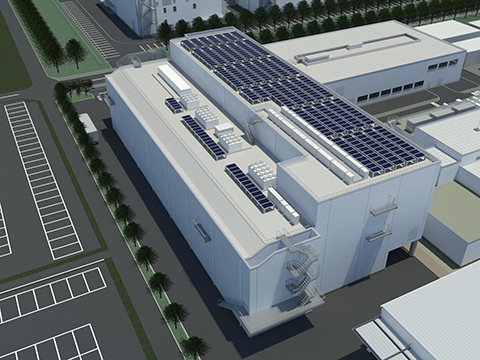
Image: Conceptual image of the completed building

The Kyowa Kirin Group companies strive to contribute to the health and well-being of people around the world by creating new value through the pursuit of advances in life sciences and technologies.
You can see this table by scrolling horizontally.
| Name | HB7 Building (tentative) |
|---|---|
| Construction method / size | Base isolated building with 1 underground floor and 4 aboveground floors; Total floor space: 8,540㎡ |
| Investment amount | Over ¥10 billion (undetermined) |
| Functions | Manufacturing of biopharmaceutical APIs, research into biopharmaceutical API manufacturing processes and staff training |
| Scheduled completion | April 2024 |
| Features |
|
- *1: GMP
- When manufacturing pharmaceuticals, it is necessary to comply with the designated quality standards and appropriately manage the manufacturing process. GMP (Good Manufacturing Practice) covers these requirements for pharmaceuticals manufacturing.
- *2: Continuous production system
- Continuous production refers to a production system in which the raw materials are continuously fed into the production process and the API is continuously produced. Continuous production is receiving increased attention as a technology for enabling stable supply, faster development and decreased manufacturing costs of pharmaceuticals.