Quality Assurance
Policy and Strategy
Pursuant to its global Quality Policy, the Kyowa Kirin Group is committed to providing products and services that earn the satisfaction and trust of its customers.
Kyowa Kirin Group Quality Policy
Revised on November 1, 2023
1. Purpose
The Kyowa Kirin Group will contribute to the health and well-being of people around the world by consistently providing world-class quality pharmaceutical products and services in compliance with our global quality, safety and compliance policies.
This policy is to describe the basic principles for quality of the Kyowa Kirin Group.
2. Scope of Application
This policy applies to all personnel including officers and employees of the Kyowa Kirin Group. The Kyowa Kirin Group will request all of our business partners and agents engaged in our products and services to act in accordance with the basic principles stated in this policy.
3. Definition of Terms
- GxP means GLP (Good Laboratory Practice), GCP (Good Clinical Practice), GMP (Good Manufacturing Practice), GQP (Good Quality Practice), GDP (Good Distribution Practice) and GVP (Good Vigilance Practice).
- Quality management system is a formalized system that documents processes, procedures, and responsibilities for achieving quality policies and objectives.
4. Basic Principles
We will act in accordance with the following basic principles.
- Compliance and Continuous Improvement
- We conduct our business in compliance with all global laws, regulations, and guidelines related to GxP. To ensure compliance, we will continuously improve our quality management system.
- Cooperation and Collaboration with Stakeholders
- We maintain sound relationships with regulatory authorities, suppliers, and contractors around the world to ensure a continuous supply of high-quality pharmaceutical products.
- Predictive and Preventive Quality Assurance
- We strive to prevent problems before they occur by using information and digital technologies to proactively identify and address risks.
- Fostering a Quality Culture
- We foster a healthy Quality Culture in our organization, openly discussing issues, and collaborating with our teams to ensure that we always act with integrity from the patient's perspective.
Governance
Our Vision and Mission
Delivering the highest Quality medicines
We contribute to patients by following our Vision and executing our Mission.
- Our Vision (the what) is to help deliver the highest quality medicines to our patients by designing and implementing a world class Quality Management System (QMS) while embracing our quality, safety and compliance policies.
- Our Mission (the how) is to achieve our goals by executing our Global Quality Roadmap which is a set of key strategic initiatives that we aim to achieve over the next few years in order to become a world class organization with respect to Quality and Compliance.
A Quality Culture is really about three things, establishing a clear vision, having a set of agreed upon values that all employees live by, and providing strong leadership. In a healthy Quality Culture you will find things like science-based approaches to decisions, sound risk management, robust data integrity, compliance, a supportive management structure, an environment of preventing issues before they arise, and a continual improvement mindset.
Our ultimate goal as part of our Global Quality Roadmap is to achieve a competitive advantage and have a world class Quality System and Quality Culture.

Jonathan Patroni
Organization for Global Quality Assurance
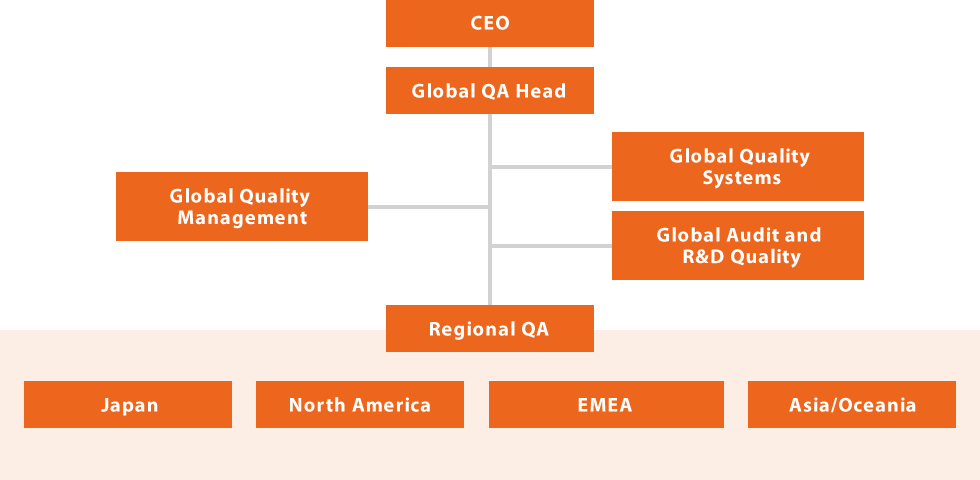
As shown in the figure below, the Global Quality Assurance function has been arranged so that the Global Quality Head reports directly to our Company Chief Compliance Officer (CCO). The Global Quality Head directly oversees all Quality Assurance activities in each region including Japan, North America, EMEA and Asia/Oceania as well as all Global QA functions. Regional QA groups work very closely with the five Global QA functions with high expertise to ensure a globally consistent structure for Quality Assurance and oversight. Our Global Quality Assurance structure has been designed and implemented with the highest priority on patient safety, compliance and customer satisfaction.
Specific Initiatives
Quality Risk Management
Kyowa Kirin regularly holds Global Product Council meetings to review global product initiatives and quality data, identify product quality issues early, and determine preventive measures to eliminate the risk of unstable supply, with patient safety as the top priority. We have an established process for escalating quality incidents to senior management and promptly convene an ad hoc Global Product Council meeting to discuss a risk-based mitigation action based on the impact and the root cause assessments.
We also take a systematic approach to the comprehensive management of drug product and drug substance quality risks by assessing, controlling, communicating and reviewing risks throughout the product lifecycle. In addition, we recognize that strengthening "predictive and preventive quality assurance" is one of our key initiatives and therefore monitor potential quality risks using quality risk registers.
Employee Quality Training
Kyowa Kirin regularly conducts quality-related training for all employees. Additionally, with November designated as Global Quality Month, we conduct activities to foster a unified quality culture and hold events in each region to raise awareness of quality and compliance.
Supplier Management
In order to ensure and maintain the level of quality we require, we conclude manufacturing and quality agreements with all of our contractors and we regularly review the status of their implementation. We also provide quality training according to the supplier's risk level and support improvement activities through regular review meetings.