Manufacturing of therapeutic antibodies
To manufacture therapeutic antibodies in a production scale, production equipments are very important. Kyowa Kirin owns a state-of-the-art factory that complies with GMP (Good Manufacturing Practice), an international standard for manufacturing control and quality control for drugs.
Manufacturing processes of therapeutic antibodies
1. Preculture
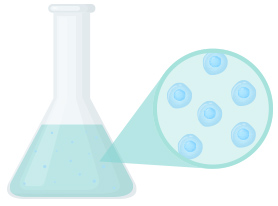
Thawing the frozen cells and culturing them for proliferation, while at the same time gradually expanding the scale of operations.
2. Main culture
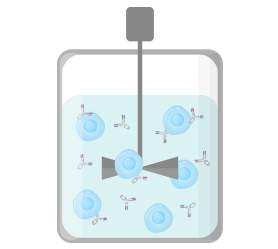
Antibodies secreted from cells accumulate in the culture medium.
The size of a culture tank (bioreactor) is about 1 to 20 tons.
3. Purification
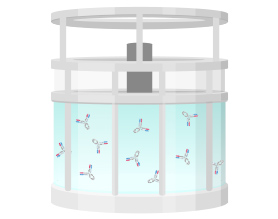
Using a centrifuge to separate cells from culture media. Then, using a filter, cell debris and the like are removed.
Using column chromatography or a special filter, only antibodies are separated from the culture after removing cells.
4. Filling
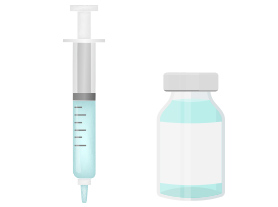
Additives are added to the purified antibodies and this mixture is filled to containers such as vials and syringes.
After undergoing a series of inspections, products are stored and transported under refrigerated conditions.
