Index
Kyowa Kirin’s advanced biopharmaceutical production technology is organized into training systems
Acquiring knowledge on the principles of biopharmaceutical production leads to success in the field
Today, the pharmaceutical market is undergoing a major shift in focus from small molecule drugs to biopharmaceuticals. As of 2020, biopharmaceuticals accounted for approximately 40% of global prescription pharmaceutical sales, with predictions that this will increase to more than 50% by 2028.* But Japanese pharmaceutical industry is plagued by a lack of engineers experienced in the production of biopharmaceuticals.
In response, Kyowa Kirin established a new Human Resources Development Office at Takasaki Plant in 2022 to train biopharmaceutical engineers. The HB7 Building, our new biopharmaceutical API manufacturing building, is scheduled for completion in March 2025 and will house a biopharmaceutical training facility with a floor space of around 800 square meters. It will offer a full range of programs combining practical training and classroom learning. We spoke to the people in charge about the human resource development system and their vision for the future.
- *
Profile
Yutaka Makimoto
Senior Manager, Human Resource Development Office, Takasaki Plant, Manufacturing Division, Kyowa Kirin Co., Ltd.
After joining the company in 1990, he was involved in the development of culture processes at the Tokyo Research Laboratory (now Tokyo Research Park), before engaging in the production of biopharmaceutical APIs for clinical trials and market launch at the Bio Process Research and Development Laboratories and at Takasaki Plant. He began working to establish the Human Resources Development Office in the General Affairs Department in 2020. He has held his current position since 2022.

Shinji Fujiwara
Human Resource Development Office, Takasaki Plant, Manufacturing Division, Kyowa Kirin Co., Ltd.
After joining the company in 1994, he was involved in the development of purification processes at the Tokyo Research Laboratory (now Tokyo Research Park), before engaging in the production of biopharmaceutical APIs for clinical trials and market launch at the Bio Process Research and Development Laboratories and at the Takasaki Plant. He has been in charge of planning, developing, and instructing educational programs related to purification processes in Takasaki Plant’s Human Resources Development Office since October 2022.

The establishment of the Human Resources Development Office at Takasaki Plant, our flagship biopharmaceutical facility

–What’s the background into the effort that has gone into strengthening human resource development at Takasaki Plant.
Mr. Makimoto Over the past few years, Kyowa Kirin has begun producing a number of global products. We have rapidly increased our workforce in response to the increase in production volume. With this has come a growing need to train new employees more rapidly and have them able to work independently as quickly as possible, as well as a need to develop middle management to properly manage the expanded organization and ensure stable operations.
–So, what was the first step?
Mr. Makimoto In addition to resolving these pressing issues, we discussed the need for a specialized department for talent development and a “human resources development philosophy” that would serve as the basis for such development, so as to continuously train people and improve the kind of talents sought by Takasaki Plant and the Takasaki Quality Unit. This led to the creation of a human resources development philosophy and the establishment of the Human Resources Development Office in October 2022. At the beginning of the discussions, the timeframe for the plan to be implemented was three years, but the plant manager called for an early implementation, noting that the development of talents was something that couldn’t wait, and that it should be done as soon as possible. The office was thus opened about six months after the decision to launch it was made.

Mr. Makimoto The Human Resources Development Office is working on the planning and operation of a training program known as the Knowledge Center, along with a dedicated training facility to be installed in the new biopharmaceutical API manufacturing HB7 Building, with the aim of fulfilling our philosophy. What stands out about this project is its simultaneous dual-sided approach, with the training program being a soft element and the training facility being a hard element.
–In the Japanese pharmaceuticals industry, a dual-sided approach incorporating both soft and hard elements is rare. Why were you able to initiate such an approach ahead of the rest of the industry?
Mr. Makimoto I think it was due to the sense of apprehension surrounding the shortage of specialized talents in biopharmaceuticals production and the increased fluidity in the workforce. There was an urgent need to create a system that would allow employees to share and advance the valuable knowledge and skills they had honed plant-wide, a move that would pave the way to growth for future generations. The desire of everyone involved, including executives, to “contribute to the bottom-up development of specialized talents industry-wide” also provided an impetus.
Kyowa Kirin’s advanced biopharmaceutical production technology is organized into training systems

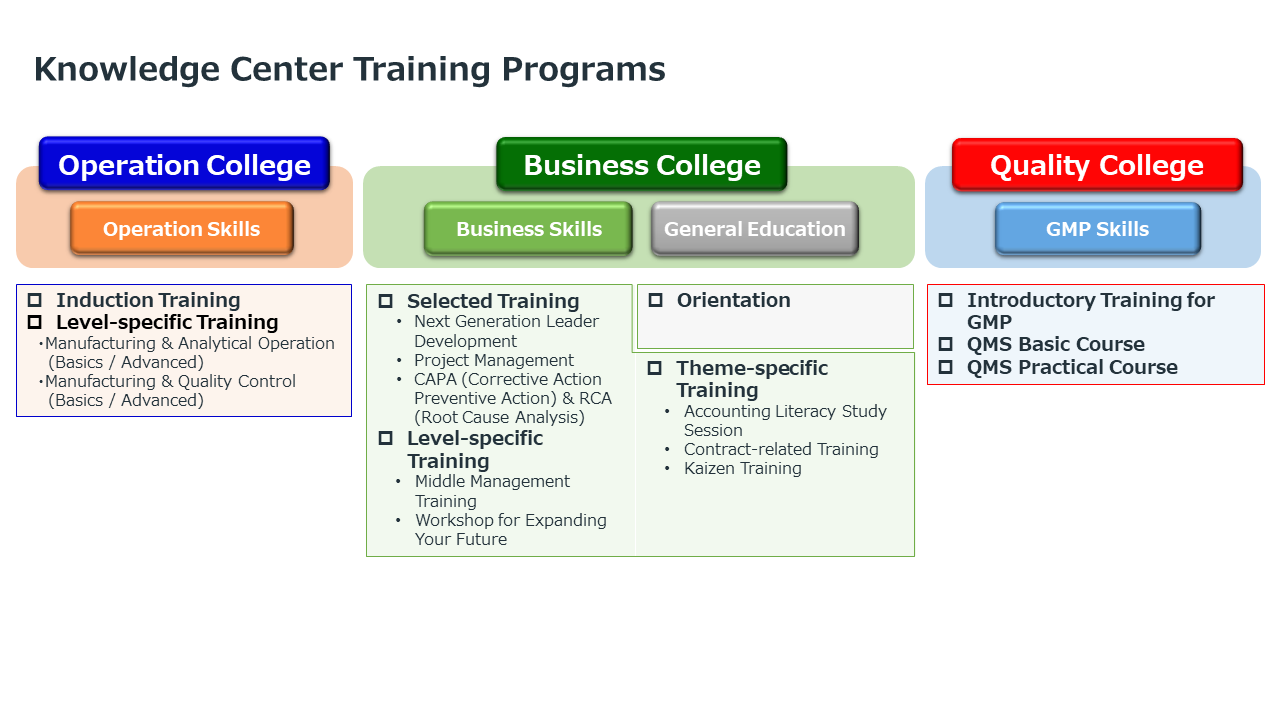
–There are three types of training systems offered by the Knowledge Center.
Mr. Fujiwara “Operation College” runs programs for acquiring operation skills. “Business College,” where business and general education is taught, offers training for those in middle management and for strengthening on-site capabilities, as well as level-specific and theme-specific training courses. “Quality College” provides basic and practical training focused on GMP (manufacturing and quality control standards). Some of these training systems are also being developed for use at other business locations.
–So, the key to training new employees lies in Operation College’s three-year training program?
Mr. Makimoto The first three years after joining our company are an important period that affects employees’ work performance. We created a systematic program with the aim of teaching all of the basic knowledge necessary for biopharmaceutical production during this period and thus cultivating human resources capable of thinking and acting independently. There are three steps to the program: training, departmental learning, and workplace practice. In training, employees are first taught the principles and mechanisms common to production operations. Next, they are separated into different departments and acquire knowledge of their respective departments, mainly via an OJT format. Finally, in the workplace practice step, they put what they have learned to practical use and acquire work skills.
Until now, the training of new employees at Takasaki Plant was conducted at their respective production buildings, which meant there was a risk that the strengths and weaknesses of each building would be passed on to the new employees being trained there. To eliminate this risk, we have brought the knowledge and experience accumulated by each building together and generalized it in the form of our human resources training program. We believe that this will allow us to pass on our engineering DNA and develop it even further.
–What kind of training actually takes place?
Mr. Fujiwara For example, the production department’s three-year training program begins with a three-week long introductory training course where trainees are shown video footage and given practical training. Over the course of the first two weeks, trainees watch some 20 video units at their own pace and learn the basics required prior to working in production. In the third week, they undergo practical training that focuses on general-purpose equipment and production techniques, including the theory behind each. Approximately 445 trainees have undertaken the program to date. The equipment used and the practical content has been kept simple, ensuring that it is easy to understand for trainees while still providing an opportunity for experienced employees to go back to the basics.

Acquiring knowledge on the principles of biopharmaceutical production leads to success in the field

–What has been the response of those that have gone through the training?
Mr. Fujiwara “I felt that the training content was well thought-out and geared toward the participants. If beginners are suddenly shown actual equipment, they’re probably going to just view it as being big and complicated; starting off with something they could see and touch, then slowly gain an understanding of the principles involved, was a refreshing way of learning, and I enjoyed it very much.” When I received this comment, I was thrilled; as a trainer, getting feedback like this is so rewarding. I also received comments such as, “Since we started with operational training on-site, learning the principles and mechanisms in the introductory training was a big help,” and “It’s not often that we get a chance to learn the basics, so this training was a great opportunity,” which has us believing that the program we are implementing is a beneficial one.
It’s not important to just do a job; what’s important is to understand the reasons we are doing it and the underlying mechanisms involved. I believe that this leads to motivation. With training, I hope to motivate participants to “learn more” and to enjoy their work.
The growing need for biopharmaceuticals ─ continuing to develop human resources with a global perspective

–Moving forward, what plans does the Human Resources Development Office have?
Mr. Fujiwara Our top priority is the establishment of the human resources base necessary for stable operations. We’d like to further enhance our training curriculum and facilities to achieve this. Not only do we want to make HB7 a training environment where employees themselves want to learn, we also want to make it a place where interdepartmental expertise generates new ideas and innovations. The development of DX human resources is also important. We hope to increase the number of people adept at using digital tools and improve business efficiency, thus investing in the future.
Mr. Makimoto In June this year, it was announced that we will be building a new biopharmaceutical plant in North Carolina. With the changes arising from this, we believe that the functions of the Human Resources Development Office and the Knowledge Center should not be confined to Takasaki Plant but should also serve as an overseas base for the development of global human resources. To that end, we would like to continue developing the Human Resources Development Office. Meanwhile, the training of new biopharmaceutical engineers is imperative for the industry as a whole. If there is a need outside our company, we would be happy to extend our activities outside Kyowa Kirin and contribute to human resources development throughout the pharmaceuticals industry.